Oncomedicine 2017; 2:37-41. doi:10.7150/oncm.17909 This volume Cite
Short Research Communication
Thrombopoietin Receptor Agonists are Effective in Treating Chemotherapy-induced Thrombocytopenia in Patients with Gliomas Undergoing Myelotoxic Treatment
1. Dept. Neurology, Barrow Neurological Institute, 240 W Thomas Rd, Phoenix, AZ 85013, USA
2. Dept. Family Medicine, Hidalgo Medical Services, 530 De Moss St, Lordsburg, NM 88045, USA
Received 2016-11-20; Accepted 2016-12-23; Published 2017-1-1
Abstract
Introduction: Chemotherapy-induced thrombocytopenia (CIT) is the principal dose-limiting toxicity in patients with glioma undergoing chemotherapy, affecting up to 25% of such patients.
Methods: This is a retrospective, unblinded case series. Patients undergoing chemotherapy for glioma (astrocytoma, oligodendroglioma or glioblastoma) who developed CIT were prescribed a thrombopoetin receptor agonist (TRA, i.e. eltrombopag or romiplostim). Doses were increased on a weekly basis, as required, until platelets were > 100×109/L or this goal was not achieved with maximum dosing. Chemotherapy was resumed if possible and patients were followed as long as they remained on treatment.
Results: A TRA was effective for CIT in 26/27 (96%). Once treated, all patients were able to resume chemotherapy as planned. Chemotherapy was continued for a median (range) of 11 months (2-28) and patients received an additional 5625 (0-16000) mg/m2 of temozolomide, 0 (0-720) mg/m2 of lomustine and 75 (0-390) mg/kg of bevacizumab. No patients in this series stopped chemotherapy due to completion of a planned regimen.
Conclusions: By using TRAs for CIT, we were able to continue chemotherapy for a longer time and at higher doses than would have been possible without this treatment. A larger series is necessary in order to determine whether this translates into improved clinical outcomes. CIT is a common problem throughout oncology, and therefore we believe that use of TRA's for this purpose should be further investigated.
Keywords: glioma, thrombopoietin, thrombocytopenia, antineoplastic agents
Introduction
CIT refers to a platelet count below the lower limit of normal due to chemotherapy. In practice, a threshold of <100×109/L is typically used. It may be divided into grades to indicate severity, as shown in Table 1. CIT is the most common reason for stopping chemotherapy (or reducing the dose) in patients with glioma. Temozolomide, bevacizumab and lomustine are typically used to treat gliomas, and all are myelotoxic.
Treatment of gliomas
Our own practice, in patients newly diagnosed with glioma where chemotherapy is prescribed, follows the Stupp regimen [1]. This uses temozolomide 75 mg/m2 po daily for 6 weeks (concurrent with radiotherapy) followed by at least 6 and ideally 12 cycles. These cycles are for 5/28 days. The first cycle is usually 150 mg/m2 and thereafter increased to 200 mg/m2. Treatment beyond 12 months is individualized.
If progression occurs, we usually add bevacizumab at 10 mg/m2 iv every 2 weeks. We change the alkylating agent from temozolomide to lomustine 100 mg/m2 po every 6 weeks, either at this time or if further progression occurs. Treatment is individualized and bevacizumab is sometimes used earlier in the course of treatment. A second course of radiotherapy with concurrent temozolomide, usually over 3 weeks, is used on occasion.
Once the platelet count drops below 100×109/L, in a patient on lomustine or temozolomide, chemotherapy dosing is typically decreased, as further decline in the platelet count is almost inevitable without modification of treatment. A threshold of 50×109/L is used with bevacizumab, which is less myelotoxic, when this agent is used alone.
Grades of severity of thrombocytopenia, as per the CTCAE [2]. LLN - lower limit of normal.
| Grade | Platelets |
|---|---|
| 1 | <LLN - 75×109 /L |
| 2 | < 75×109 /L - 50×109 /L |
| 3 | < 50×109 /L - 25×109 /L |
| 4 | < 25×109 /L |
Prevalence of CIT
25% of patients on temozolomide monotherapy developed CIT in the largest series to date which addresses this question (n=101) [3]. Cumulative dosing of temozolomide was the only variable studied associated with CIT. Platelet counts of <50 occurred in 8% of patients.
20% were affected by counts of <50 in a series of 52 patients [4]. In 80% of those affected, toxicity occurred during, or soon after, initial concurrent chemo-radiotherapy. 90% of sufferers stopped chemotherapy due to CIT.
10-11% of those in clinical trials of glioblastoma using temozolomide developed CIT; such patients tend to have a better performance status than an unselected group of patients with glioma [5, 6].
Bevacizumab, when added to a variety of cancer regimens is associated with a higher rate of CIT (relative risk 1.22; p=0.047) [7].
44-54% of those using lomustine in clinical trials of glioma treatment develop platelets of < 50. [8, 9]. When lomustine is combined with bevacizumab, the development of platelets of <50 has been reported to be dose-dependent, affecting 63% of patients taking lomustine 110 mg/m2 po q6 wks vs. 9% of patients on lomustine 90 mg/m2 [10].
Materials and Methods
Ethical approval for this work was obtained from the Institutional Review Board of St. Joseph's Hospital.
We reviewed patients who had received a TRA in our practice over the period Jul 2014 to Dec 2015 while receiving chemotherapy for glioma (WHO grades II, III and IV). Treatment was instigated at the discretion of the treating physician but was typically employed when platelet counts had fallen below 100. None of these patients were using forms of chemotherapy apart from the three already highlighted. With one exception (discussed below) none had other factors predisposing to thrombocytopenia.
The TRA eltrombopag was used when possible, as oral administration was preferable for patients. However, treatment decisions were typically dependent on insurer preference and romiplostim (administered subcutaneously) was used in some cases. Dosing was based on existing guidelines for the use of TRAs in the treatment of immune thrombocytopenia. Once platelet counts reached >100, we attempted to resume chemotherapy as planned.
We recorded the time from starting chemotherapy to the time of the nadir of platelet counts, the value of the count at that time and the cumulative dosing of chemotherapy. We recorded time until last observation, treatment effectiveness, outcome (treatment ongoing, hospice care or death) as well as the additional cumulative chemotherapy given since the platelet nadir.
Analysis was performed using R and is given as Supplementary Material [11, 12]. Herein, for continuous variables, results are generally given as median [interquarile range (IQR)]. Time-to-event data is given as a median with a 95% confidence interval (NA indicates not applicable, as insufficient numbers of events had occurred).
Results and Discussion
40 patients were prescribed a TRA over the 18 months reviewed. Of these, 13 did not start treatment, typically due to lack of insurance coverage and in some cases due to progressive disability resulting in cessation of chemotherapy. These cases are not reviewed further here.
Table 2 shows the 27 individual patients and summary statistics for each variable; the complete dataset is part of the Supplementary Material.
Nadir
The lowest platelet count was 69 [46-86] when a TRA was instituted. This nadir was reached 4.4 [2.2 - 12] months after starting chemotherapy; the nadir occurred within 3 months of starting treatment in 9/27 (33%) patients.
Cumulative doses of chemotherapy prior to developing CIT and thereafter until the last follow-up are shown in Table 3. When the nadir was reached, 41% had used at least some bevacizumab and 19% at least some lomustine.
Eltrombopag was used in 22/27 (81%) of patients; the others received romiplostim. Platelets of >100 were restored in most cases with the standard starting dose of the TRA: for eltrombopag, 17/22 (77%) with 50 mg daily; for romiplostim 4/5 (80%) with 1 microg/kg/week.
Subsequent treatment
Nearly all patients were able to continue treatment as originally planned. The sole exception (Table 2, ID=26) was a survivor of childhood leukemia (AML) who had received systemic chemotherapy at that time. Pancytopenia, with platelets of 7, developed soon after initial chemo-radiotherapy. Little improvement was seen despite maximal dosing of a TRA. Thus, no further chemotherapy was possible.
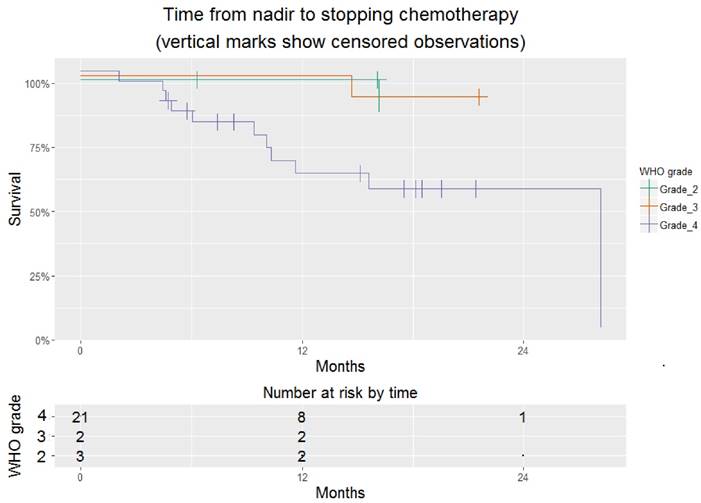
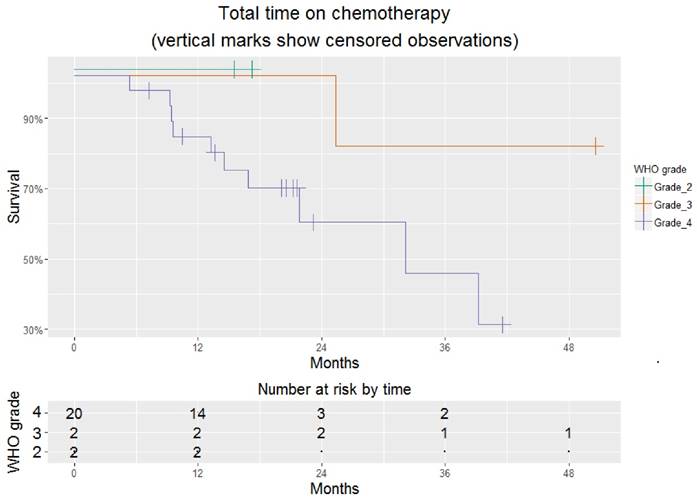
Excluding this individual, the time to stopping chemotherapy (hospice care or death) was 15.6 [10.3 - NA] months. At the time of last follow-up, 14/27 (52%) had stopped chemotherapy (Figure 1). Overall time on chemotherapy was encouraging, particularly for those with glioblastoma, whose time on chemotherapy was 32.3 [16.9 - NA] months (Figure 2).
Patients receiving a TRA. Last row shows summary statistics, given as percentages or median [interquartile range].
| ID | WHO grade | Age | Sex | Platelet nadir | Months to nadir | Cumulative, before nadir | T | D | E | Months to last f/up | O | Additional cumulative | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tem | bev | lom | tem | bev | lom | |||||||||||
| 1 | 3 | 58 | m | 98 | 10.68 | 6900 | 170 | 0 | e | 25 | y | 14.66 | d | 6750 | 50 | 0 |
| 2 | 4 | 58 | m | 85 | 11.00 | 9900 | 70 | 0 | e | 50 | y | 28.18 | h | 9000 | 320 | 0 |
| 3 | 4 | 72 | f | 41 | 5.00 | 3900 | 0 | 0 | e | 50 | y | 4.6 | h | 5000 | 0 | 0 |
| 4 | 4 | 41 | f | 58 | 19.82 | 4425 | 60 | 300 | e | 50 | y | 2.04 | h | 2000 | 30 | 0 |
| 5 | 4 | 72 | m | 64 | 3.62 | 3900 | 0 | 0 | e | 200 | y | 19.53 | o | 6000 | 240 | 0 |
| 6 | 4 | 73 | m | 62 | 0.03 | 0 | 0 | 0 | e | 50 | y | 9.4 | h | 9850 | 60 | 0 |
| 7 | 4 | 57 | m | 69 | 20.12 | 17900 | 320 | 280 | e | 50 | y | 21.4 | o | 0 | 300 | 720 |
| 8 | 3 | 44 | f | 77 | 28.96 | 23475 | 100 | 0 | e | 50 | y | 21.57 | o | 8000 | 260 | 500 |
| 9 | 4 | 49 | f | 18 | 26.14 | 17900 | 30 | 200 | e | 50 | y | 6.02 | h | 0 | 45 | 200 |
| 10 | 4 | 35 | f | 61 | 1.61 | 3150 | 0 | 0 | r | 1 | y | 18.48 | o | 9750 | 110 | 100 |
| 11 | 4 | 65 | f | 5 | 1.28 | 1575 | 0 | 0 | r | 1 | y | 15.62 | h | 0 | 60 | 0 |
| 12 | 4 | 62 | f | 50 | 1.94 | 3150 | 0 | 0 | e | 50 | y | 18.15 | o | 10750 | 230 | 0 |
| 13 | 4 | 39 | m | 75 | 4.14 | 5400 | 0 | 0 | e | 50 | y | 17.49 | o | 16000 | 390 | 0 |
| 14 | 2 | 26 | f | 86 | 211.10 | 33775 | 80 | 100 | e | 50 | y | 16.14 | h | 0 | 80 | 100 |
| 15 | 4 | 60 | f | 94 | 1.64 | 3150 | 0 | 0 | e | 50 | y | 11.61 | h | 12525 | 230 | 300 |
| 16 | 2 | 38 | f | 79 | 1.18 | 3150 | 0 | 0 | r | 10 | y | 16.08 | o | 5250 | 60 | 0 |
| 17 | 4 | 71 | f | 81 | 4.37 | 4650 | 20 | 0 | e | 50 | y | 4.9 | h | 5000 | 100 | 0 |
| 18 | 4 | 70 | f | 98 | 0.95 | 3150 | 0 | 0 | r | 1 | y | 4.44 | h | 2000 | 0 | 0 |
| 19 | 4 | 63 | m | 95 | 5.42 | 5900 | 0 | 0 | e | 50 | y | 15.16 | o | 13000 | 0 | 0 |
| 20 | 4 | 46 | m | 40 | 71.47 | 56700 | 195 | 440 | e | 50 | y | 10.06 | h | 0 | 195 | 700 |
| 21 | 4 | 74 | f | 25 | 7.89 | 9900 | 0 | 0 | e | 150 | y | 5.72 | o | 5000 | 0 | 0 |
| 22 | 4 | 64 | m | 64 | 4.24 | 5900 | 10 | 0 | e | 50 | y | 10.32 | h | 0 | 250 | 400 |
| 23 | 2 | 51 | f | 34 | 9.21 | 6900 | 0 | 0 | e | 50 | y | 6.31 | o | 7000 | 0 | 0 |
| 24 | 4 | 44 | f | 73 | 12.95 | 10900 | 50 | 0 | e | 50 | y | 8.28 | o | 9000 | 190 | 0 |
| 25 | 4 | 63 | f | 92 | 3.06 | 4150 | 0 | 0 | r | 1 | y | 7.4 | o | 7000 | 0 | 0 |
| 26 | 3 | 37 | f | 7 | 2.40 | 3150 | 0 | 0 | e | 150 | n | 3.85 | h | 0 | 0 | 0 |
| 27 | 4 | 69 | m | 88 | 2.53 | 3150 | 0 | 0 | e | 100 | y | 4.73 | o | 1500 | 70 | 0 |
| 2: 11% 3: 11% 4: 78% | 58 [44-67] | f: 63% | 69 [46-86] | 4.4 [2.2-12] | 4650 [3150-9900] | 0[0-55] | 0[0-0] | e: 81% | y: 96% | 11 [6.1-17.2] | o: 48% | 5625 [1625-9000] | 75 [34-230] | 0[0-100] | ||
Abbreviations: tem - temozolomide; bev - bevacizumab; lom - lomustine. Cumulative dosing of chemotherapy is given in the following units: tem, lom - mg/m2; bev - mg/kg. T - treatment: e - eltrombopag; r - romiplostim. D - dose: eltombopag, 25 - 200 mg po daily; romiplostim, 1 -10 microg/kg subcut weekly. E - effective ? (i.e. platelets returned to >100×109 /L). O - outcome: d -died; h - hospice care; o - treatment ongoing.
Time from platelet nadir to hospice referral or death for 26 patients treated successfully with a TRA. This is stratified by WHO grade. Where survival=100% for all grades, lines are jittered to avoid overlap.
Total time on chemotherapy for 26 patients treated successfully with a TRA. This is stratified by WHO grade. Where survival=100% for all grades, lines are jittered to avoid overlap.
Cumulative doses of chemotherapy received before and after starting a TRA. Values are median [IQR]. As an example, temozolomide 200 mg/m2 for 5/28 days for 3 cycles = 3000 mg/m2.
| temozolomide mg/m2 | bevacizumab mg/kg | lomustine mg/m2 | |
|---|---|---|---|
| Before CIT | 4650 [3150-9900] | 0 [0-55] | 0 [0-0] |
| After CIT | 5625 [1625-9000] | 75 [34-230] | 0 [0-100] |
Side effects
One patient had intractable itching after each dose of eltrombopag, which lasted 2-3 hr. These symptoms were tolerable once dosing was changed to twice/week and treatment continued as planned thereafter. There was one sudden unexpected death (Table 1, ID=1); we suspect this was a pulmonary embolism although no autopsy was performed.
Discussion
As shown, CIT was remediated in all patients but one and did not re-occur despite prolonged subsequent chemotherapy. CIT should be distinguished from the rarer aplastic anemia associated with temozolomide, as in our patient who did not respond to treatment [13].
Romiplostim has already been shown to be safe and effective in CIT in a series of 20 patients undergoing chemotherapy for a variety of cancers, including one patient with glioblastoma [14]. Of those who resumed chemotherapy, 14/15 (93%) tolerated at least two additional cycles and 4/15 (27%) continued treatment for an additional year or more.
Currently, the only FDA-approved agent for CIT is oprelvekin (IL-11) [15]. This requires daily subcutaneous dosing. The improvement in platelet counts tends to be less marked and side effects more common than with the TRAs.
Conclusions
Being that CIT is such a common problem in oncology, we encourage our colleagues to investigate TRAs as part of supportive care for other myelotoxic regimens. Given their effectiveness, a randomized trial may be hard to justify [16]. Without FDA approval for CIT, lack of insurance coverage may be a barrier to their use, at least in the USA.
Supplementary Material
Statistics.
Abbreviations
CIT: chemotherapy-induced thrombocytopenia; TRA: thrombopoetin-receptor agonist; IQR: interquartile range.
Competing Interests
The authors have declared that no competing interest exists. No financial or material support was received for this work.
References
1. Stupp R Mason WP, van den Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96 PMID: 15758009
2. US department of health and human services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Revised 14 June 2010. http://evs.nci.nih.gov/ftp1/CTCAE/
3. Simó M, Velasco R, Graus F. et al. Impact of antiepileptic drugs on thrombocytopenia in glioblastoma patients treated with standard chemoradiotherapy. J Neurooncol. 2012;108(3):451-8 PMID: 22407174
4. Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol. 2007;9(1):47-52 PMID: 17108062
5. Stupp R, Dietrich PY, Ostermann Kraljevic S. et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20(5):1375-82 PMID: 11870182
6. Stupp R, Mason WP, van den Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96 PMID: 15758009
7. Schutz FA, Jardim DL, Je Y, Choueiri TK. Haematologic toxicities associated with the addition of bevacizumab in cancer patients. Eur J Cancer. 2011;47(8):1161-74 PMID: 21470847
8. Wick W, Puduvalli VK, Chamberlain MC. et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168-74 PMID: 20124186
9. Walker MD, Alexander E Jr, Hunt WE. et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333-43 PMID: 355604
10. Taal W, Oosterkamp HM, Walenkamp AM. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943-53 PMID: 25035291
11. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013
12. Dardis C, Woolf EC, Scheck AC. Towards reproducible research: From data analysis (in R) to a typeset laboratory notebook (as. pdf) using the text editor Emacs with the'mp'package. F1000Research. 2015:4 DOI: 10.12688/f1000research.6800.2
13. Federal Drug Administration (FDA). Drug Safety Newsletter. Postmarketing Reviews. 2007;1(1):7
14. Parameswaran R, Lunning M, Mantha S. et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22(5):1217-22 PMID: 24414994
15. Bhatia M, Davenport V, Cairo MS. The role of interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with solid tumors, lymphoma, acute myeloid leukemia and bone marrow failure syndromes. Leuk Lymphoma. 2007;48(1):9-15 PMID: 17325843
16. Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459-61 PMID: 14684649
Author contact
![]() Corresponding author: christopherdardiscom, Office 602 406 6238, Fax 602 406 6260
Corresponding author: christopherdardiscom, Office 602 406 6238, Fax 602 406 6260