Oncomedicine 2018; 3:28-36. doi:10.7150/oncm.22614 This volume Cite
Research Paper
Overexpression of EGFR in Oral Premalignant Lesions and OSCC and Its Impact on Survival and Recurrence
1. Department of Surgery, Aga Khan University Hospital, Karachi, Pakistan
2. Department of Pathology and Laboratory Medicine, Aga Khan University Hospital, Karachi, Pakistan
Received 2017-8-30; Accepted 2017-10-14; Published 2018-4-27
Abstract
Introduction: Oral squamous cell carcinoma (OSCC) the sixth leading cancer worldwide ranks as the most common cancer in males, and the third most common in females in Pakistan. It is influenced by risk factors which are widely consumed in our population. The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that is imperative for cell signalling, growth and differentiation. It is mutated and overexpressed in a variety of cancers, while in OSCC it has been linked to poor patient survival; premalignant to malignant transformation and recurrence. This study investigates the use of EGFR as a prognostic factor for OSCC.
Materials and Methods: Premalignant (n=29) and OSCC (n=100) formalin-fixed paraffin-embedded tissues were retrieved from the surgical archives of Aga Khan University Hospital (AKUH). Immunohistochemistry for EGFR overexpression was performed using monoclonal antibody on both groups. EGFR expression was correlated with habits of risk factor consumption, clinicopathologic features and 5-year survival and recurrence.
Results: 15/29 premalignant and 67/100 OSCC patients had overexpressed EGFR. The upper/lower lip had the highest EGFR positivity among all premalignant sites of lesion (p=0.041). In OSCC patients, those who had EGFR overexpression had worse 5-year survival (univariate: p=0.048, multivariate: p=0.056) and higher chances of recurrence (univariate: p=0.01, multivariate: p=0.004) as compared to EGFR negative patients.
Conclusion: EGFR is a viable candidate for an OSCC prognostic marker since its overexpression leads to poor survival and markedly increases the chances of recurrence.
Keywords: 5year survival, EGFR, immunohistochemistry, oral premalignant lesions, OSCC
Introduction
Oral cancer makes up 3-4% of all cancers worldwide and is currently the sixth most common cancer. The oral squamous cell carcinoma (OSCC) is the most frequent type and comprises 90% of all malignancies of the oral cavity [1]. Globally in the year 2012, the highest rates for lip and oral cavity cancers were found in South-Central Asia, Central and Eastern Europe and Melanesia; whereas Western Africa and Eastern Asia boasted the lowest incidences. Cancers of the oral cavity and pharynx together made up 52900 new cases and were responsible for 292000 deaths [2]. The survival of OSCC patients is bleak with a 5-year survival rate of 40-50% [3] which is in part due to patients presenting with advanced disease at the time of diagnosis and partly due to inadequate treatment and disease management strategies. Moreover, 50% of patients are seen to develop recurrence within the first 2 years of treatment leading to a worse prognosis [4]. It was reported by Camisasca et al. that 5 year survival rates for OSCC patient with and without recurrence were 30% and 92% respectively [5].
Pakistan is considered one of the high-risk regions of the world for head and neck cancers [6]. GLOBOCAN figures for 2012 reveal that lip and oral cavity cancers are the second-most common cancer in Pakistan with 4,046 deaths in males and 3,220 deaths in females attributed to lip and oral cavity cancers [7]. Lip and oral cavity cancers ranked as the most common type of cancer for Pakistani males and the third most common type for Pakistani females, being the second most common cancer overall [8, 9]. The world's highest incidence for oral cavity cancer has been reported in Karachi [10, 11] and this is believed to be due to the habitual and widespread use of various OSCC risk factors. An increasing incidence of oral cancer among the youth has also been noted with a 60% increase in the number of patients below the age of 40 over the past 30 years [12].
Several risk factors have been identified as key players in the development and progression of OSCC such as tobacco smoking [7], smokeless tobacco [13] alcohol [14], betel quid [15] and areca nut chewing [16] and HPV infection [17]. Additionally, an equally common use of risk factors has been observed in both genders in Pakistan. This may be a reason for the equally high prevalence of oral cancer in both sexes which is a statistic not observed in other regions of the world. Moreover, alcohol has not been implicated as a significant risk factor in the population of Karachi, due to lower consumption as compared to the west. Whereas tobacco, areca nut and betel quid in the cultural forms of paan, supari, gutka, niswar and chalia is much more common [18]. A recent survey revealed that 36% of males and 44% of females consumed paan alone or in conjunction with tobacco and this correlated with the high prevalence of oral cavity cancers [12].
The exact cause of OSCC is unknown; however, many genetic mutations are thought to be involved in its development and progression. Prominent genes believed to have a role in the carcinogensis of OSCC are TP53, NOTCH 1, EGFR, CDKN2A, STAT3, Cyclin D1 and Rb [19]. The epidermal growth factor regulator (EGFR) is a transmembrane growth factor receptor with a molecular weight of 170 kDaltons which binds with many ligands including epidermal growth factor, amphiregulin, betacellulin, TGF- α and others. Binding of any of these ligands to the receptor induces conformational changes resulting in intrinsic tyrosine kinase activity which activates multiple signalling pathways that regulate cell proliferation, differentiation, motility and survival [20].
Oral premalignant lesions precede oral carcinomas in about 67% of cases, with leukoplakia being the most common lesion. Statistically speaking, 1%-18% of these premalignant lesions will undergo transformation into malignancy [21]. EGFR has been associated in premalignant lesions in two ways: Firstly, gene amplification of EGFR was observed to help transformation of premalignant lesions into malignant oral conditions by Benchekroun et al. [22]. Secondly, Poh et al. found that increased EGFR gene gain of any level (high or low) had a strong association with progression into malignancy and aggressive clinical behaviour [23].
The impact of EGFR on survival and pattern of relapse was tested in a correlative study of HNSCC patients and it was seen that patients with overexpressed EGFR had significantly lower overall survival and disease free survival rates. It was concluded that EGFR was a significant independent determinant of survival and a strong predictor for locoregional relapse [24]. Various other cancers have also been found to harbour EGFR mutations and overexpression such as glioblastoma multiforme [25], lung cancer [26], head and neck squamous cell carcinoma [27], breast [28], colorectal [29] and oesophageal cancers [30] leading to a worse patient prognosis.
A study utilized next-generation sequencing and identified EGFR as one of the most commonly mutated cancer-related gene in OSCC [31] and it is also considered a major player in oral carcinogenesis [32] as it was found to harbour genetic alterations as a result of repeated exposure to risk factors [19]. According to Gupta et al., EGFR overexpression lead to lower overall survival, overall response and quality of life in patients with locally advanced OSCC who had been treated with chemoradiation [33]. Furthermore, a meta-analysis of thirty-seven studies was conducted by Keren et al. to analyse the prognostic value of EGFR in HNSCC and it revealed that EGFR overexpression to lead to lower survival and disease-free survival for oropharyngeal carcinoma [34].
To the best of our knowledge; no studies concerning the role of EGFR in OSCC in the Pakistani population have been performed before. The evidence for the impact of EGFR on survival and recurrence deserves to be explored in the Pakistani population as it suffers from a record high prevalence of OSCC and patients encounter inadequate diagnostic and treatment measures. EGFR has the potential to become a target for therapeutic measures in the form of monoclonal antibodies or tyrosine kinase inhibitors as has been the case for breast [28], colorectal and lung cancer [35]. As a result, improvement in patient prognosis and overall survival may be seen. Our objective for this study was to determine EGFR expression in premalignant and OSCC patients of Pakistan and explore its impact on 5-year survival and recurrence in OSCC patients.
Materials and Methods
Study Population
The study was a retrospective series in which patients who had been diagnosed and treated at Aga Khan University Hospital (AKUH), Karachi, Pakistan from the year 1991 to 2004 were included, amounting to a total of 29 premalignant and 100 OSCC patients. All patients provided written informed consent for their participation. Ethical approval was obtained prior to the start from the Ethical Review Committee of AKUH. Patients who did not have complete medical records, 5-year follow up data, had inadequate samples or those who had undergone treatment beyond AKUH were excluded. For each case, the following information was obtained: age, gender, site of primary lesion, type of lesion, histological differentiation, AJCC and TNM stages, habit history (meaning use of any risk factors such as tobacco, betel quid or areca nut) and surgical margin status.
EGFR Immunohistochemical Assay
Formalin-fixed paraffin embedded blocks of selected patients were retrieved from the histopathology laboratory of AKUH. 4µm thick sections of each tissue block were sliced onto precoated glass slides and heated in an oven at 55ºC for an hour. Sections were deparaffinised using xylene, dehydrated in a graded ethanol series and rinsed with distilled water. The staining procedure for EGFR was performed using EnVision FLEX, Dako. Proteinase K was used for antigen retrieval for 10 minutes at room temperature, followed by Peroxidase-Blocking Reagent for 10 minutes. Slides were incubated with Mouse Monoclonal Anti-Human EGFR Clone H11 diluted 1:200 with buffer and incubated for an hour. Subsequently, HRP/FLEX was applied for an hour and visualization was achieved using chromogen diaminobenzidine diluted 2 drops in 1000ml substrate buffer for 30 seconds with hematoxylin serving as counterstain. Negative and positive controls were processed alongside each batch.
Evaluation of EGFR expression
All slides were evaluated independently by two pathologists who were unaware of the clinical diagnosis to each other's evaluation. Any discrepancies arising between them were resolved using a conference microscope. To account for varying antigen expression within the tumour, 2-6 different visual fields were observed at 20x and 40x. EGFR positivity was expressed as membranous and/or cytoplasmic brown colour deposition. Scoring for EGFR expression was as follows: negative (<10% positive-stained cells), mild (10-20% positive-stained cells), moderate (20-60% positive-stained cells) and strong (>60% positive-stained cells).
Statistical Analysis
Statistical analysis was performed using SPSS version 22 for Windows (SPSS Inc., Chicago, IL, USA). Cross-tabulations were run using chi-square test or Fisher's exact test wherever necessary. Survival analysis was performed using the Kaplan-Meier and log-rank test. Any variables that were significant on the univariate analysis were further on multivariate. For all tests, p values <0.05 were considered significant.
Results
Premalignant patients
The premalignant study population consisted of 29 patients, 17 males (59%) and 12 females (41%) with a mean age of 43.45 (SD±20.56). Table 1 lists the characteristics of premalignant patients. EGFR expression was observed in 15 (52%) of cases. Mean age for EGFR negative and positive groups was 45.14 (SD±19.34) and 41.87 (SD±22.20) respectively. EGFR overexpression had a significant correlation with site of lesion (p=0.041) as EGFR positive patients had upper/lower lip as most common lesion, whereas cheek was more common in the EGFR negative group. The majority of EGFR positive cases were mild positives with few moderate and no strong positives.
OSCC patients
The OSCC study population consisted of 100 patients, with 56 males and 44 females having a mean age of 52.57 (SD±12.75). The most common primary site of lesion was the cheek, 65% followed by tongue, 35%. Maximum cases of tongue were of the anterior, 32% rather than posterior, 6%. Secondary sites included 27 cases of mandible, 12 of retromandible, 9 of palate, 6 involving floor of the mouth and 1 of tonsil. Skin involvement was seen in 4 patients. The greater number of patients (56%) had moderately differentiated and AJCC stage II (30%) OSCC. After a follow up of 5 years, 44 of the total 100 patients had died and 65 had a recurrence of disease. EGFR overexpression was positive in 67 cases. Table 2 lists correlations of patient characteristics and EGFR status. EGFR was significantly associated with habits (p=0.039), recurrence (p=0.004) and also marginally with living status (p=0.053). EGFR expression was seen in 4/6 and 25/32 cases of posterior and anterior tongue respectively.
Correlation of Premalignant EGFR Positive and Negative Patients with clinicopathologic factors (n=29)
Factors | Total (n=29) | EGFR Expression | Chi Square / Fisher's Exact Test | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n=15, 51.7%)) | Negative (n=14, 48.3%) | ||||||||
| N | % | N | % | N | % | ||||
| Gender | Male | 17 | 58.6 | 9 | 60.0 | 8 | 57.1 | 0.024 | 0.87 |
| Female | 12 | 41.4 | 6 | 40.0 | 6 | 42.9 | |||
| Site of Lesion | Upper/Lower Lip | 10 | 34.5 | 7 | 46.8 | 3 | 21.5 | 23.711* | 0.041** |
| Cheek | 7 | 24.1 | 2 | 13.3 | 5 | 35.7 | |||
| Gingiva | 5 | 17.2 | 2 | 13.3 | 3 | 21.5 | |||
| Tongue | 3 | 10.4 | 2 | 13.3 | 1 | 7.1 | |||
| Soft/Hard Palate | 3 | 10.4 | 2 | 13.3 | 1 | 7.1 | |||
| Tonsil | 1 | 3.4 | 0 | 0.0 | 1 | 7.1 | |||
| Diagnosis | Squamoproliferative Lesion | 6 | 20.8 | 2 | 13.3 | 4 | 28.6 | 15.74* | 0.887 |
| Hyperplastic Squamous Mucosa | 3 | 10.3 | 2 | 13.3 | 1 | 7.1 | |||
| Extravasation Mucocele | 4 | 13.8 | 3 | 20.0 | 1 | 7.1 | |||
| Retention Cyst | 2 | 6.9 | 1 | 6.7 | 1 | 7.1 | |||
| Others | 15 | 51.7 | 7 | 46.7 | 7 | 50.0 | |||
| EGFR Staining Score | Mild | 11 | 37.9 | ||||||
| Moderate | 4 | 13.8 | |||||||
| Negative | 14 | 48.3 | |||||||
*Fishers' exact test applied
**p-value significant at <0.05
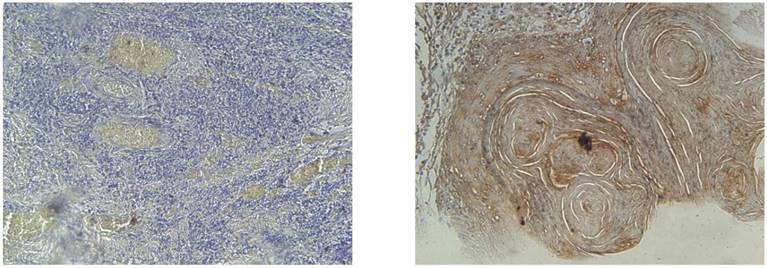
IHC results for EGFR at magnification of 40x. EGFR negative (left) and EGFR strong positive (right) with >60% of cells showing membraneous and cytoplasmic staining
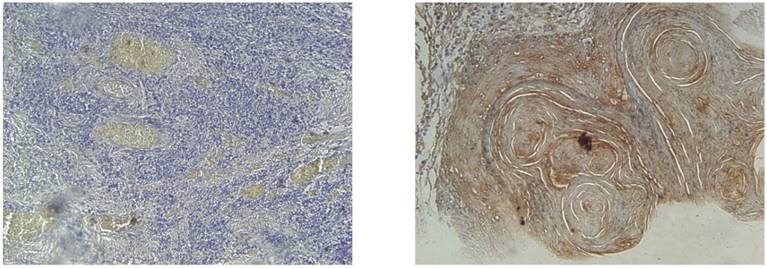
Kaplan-Meier disease free survival (left) and overall survival (right) curves in 100 OSCC patients selected according to EGFR status
Correlation of clinicopathologic factors with EGFR expression of OSCC patients (n=100)
Factors | Group | Total (n=100) | EGFR Expression | Chi-square/ Fishers Exact Test | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive (n=67) | Negative (n=33) | ||||||||||
| N / % | N | % | N | % | |||||||
| Gender | Male | 56 | 37 | 55.2 | 19 | 57.6 | 0.05 | 0.824 | |||
| Female | 44 | 30 | 44.8 | 14 | 42.4 | ||||||
| Age Division | < 40 years | 16 | 11 | 16.4 | 5 | 15.2 | 0.026 | 0.871 | |||
| ≥40 years | 84 | 56 | 83.6 | 28 | 84.8 | ||||||
| Histological Classification | Well Differentiated | 41 | 25 | 37.3 | 16 | 48.5 | 1.857* | 0.379 | |||
| Moderately Differentiated | 56 | 39 | 58.2 | 17 | 51.5 | ||||||
| Poorly Differentiated | 3 | 3 | 4.5 | 0 | 0.0 | ||||||
| AJCC Stage | I | 18 | 9 | 13.4 | 9 | 27.3 | 7.067 | 0.07 | |||
| II | 30 | 18 | 26.9 | 12 | 36.4 | ||||||
| III | 27 | 23 | 34.3 | 4 | 12.1 | ||||||
| IV | 25 | 17 | 25.4 | 8 | 24.2 | ||||||
| Habits | Yes | 73 | 46 | 68.7 | 27 | 81.8 | 6.23* | 0.039** | |||
| No | 19 | 17 | 25.4 | 2 | 6.1 | ||||||
| Unknown | 8 | 4 | 6.0 | 4 | 12.1 | ||||||
| Tobacco/ Smoking | Yes | 34 | 21 | 31.3 | 13 | 39.4 | 2.571 | 0.277 | |||
| No | 41 | 26 | 38.8 | 15 | 45.5 | ||||||
| NA | 25 | 20 | 29.9 | 5 | 15.2 | ||||||
| Paan/Supari | Yes | 57 | 34 | 50.7 | 23 | 69.7 | 3.526 | 0.172 | |||
| No | 18 | 13 | 19.4 | 5 | 15.2 | ||||||
| NA | 25 | 20 | 29.9 | 5 | 15.2 | ||||||
| Chalia/Gutka/Niswar | Yes | 27 | 16 | 23.9 | 11 | 33.3 | 2.76 | 0.277 | |||
| No | 48 | 31 | 46.3 | 17 | 51.5 | ||||||
| NA | 25 | 20 | 29.9 | 5 | 15.2 | ||||||
| Primary site | Cheek | 65 | 41 | 61.2 | 24 | 72.7 | 1.293 | 0.256 | |||
| Tongue | 35 | 26 | 38.8 | 9 | 27.3 | ||||||
| Primary margins | Clear | 62 | 40 | 29.7 | 22 | 66.7 | 0.872* | 0.708 | |||
| Near | 30 | 22 | 32.8 | 8 | 24.2 | ||||||
| Involved | 8 | 5 | 7.5 | 3 | 9.1 | ||||||
| Final T stage | T1 | 21 | 11 | 16.4 | 10 | 30.3 | 5.1* | 0.161 | |||
| T2 | 44 | 29 | 43.3 | 15 | 45.5 | ||||||
| T3 | 16 | 14 | 20.9 | 2 | 6.1 | ||||||
| T4 | 19 | 13 | 19.4 | 6 | 18.2 | ||||||
| Final N stage | N0 | 75 | 48 | 71.6 | 27 | 81.8 | 2.2* | 0.305 | |||
| N1 | 17 | 14 | 20.9 | 3 | 9.1 | ||||||
| N2 | 8 | 5 | 7.5 | 3 | 9.1 | ||||||
| Radiotherapy | Yes | 58 | 37 | 55.2 | 21 | 63.6 | 2.56* | 0.299 | |||
| No | 32 | 21 | 31.3 | 11 | 33.3 | ||||||
| Unknown | 10 | 9 | 13.4 | 1 | 3.0 | ||||||
| 5 year suvival | Alive | 56 | 33 | 49.3 | 23 | 69.7 | 3.75 | 0.053** | |||
| Dead | 44 | 34 | 50.4 | 10 | 30.3 | ||||||
| Recurrence | Yes | 65 | 50 | 74.6 | 15 | 45.5 | 8.271 | 0.004** | |||
| No | 35 | 17 | 25.4 | 18 | 54.5 | ||||||
| EGFR Staining Intensity | Negative | 33 | |||||||||
| Mild | 11 | ||||||||||
| Moderate | 41 | ||||||||||
| Strong | 15 | ||||||||||
*Fishers' exact test applied
**p-value considered significant at <0.05
AJCC: American Joint Commission on Cancer
5 year survival and EGFR overexpression
Living status was taken as the time interval from primary treatment until death or last follow-up taken at 5 years (60 months. Mean 5 year survival time for our population was 43 months with an overall survival rate of 56%. Univariate analysis showed that AJCC stage (p=0.004), final N stage (p=<0.001), EGFR expression (p=0.048) and EGFR staining intensity (p=0.015) all had a significant effect on 5 year survival. Moreover, EGFR expression (p=0.056) and AJCC stage (p=0.003) were also proven to be independent determiners of 5 year survival on the multivariate analysis (Table 3A and Table 3B).
Disease free survival and EGFR overexpression
Recurrence was defined as any relapse, locoregional or distant; however in our data there was no distant metastasis. Overall mean disease free survival time was 82 months with a recurrence rate of 65%. Univariate analysis revealed AJCC stage (p=0.01, final N stage (p=<0.001), EGFR expression (p=0.01) and EGFR staining intensity (p=0.01) to be significantly associated with recurrence of patients. However, on the multivariate analysis only final N stage (p=<0.001) and EGFR expression (p=0.004) had a strong independent influence on disease free survival (Table 3A and Table 3B).
Discussion
Pakistan suffers from a very high prevalence of OSCC and the city of Karachi has been reported to have one of the highest incidences of OSCC worldwide [11, 36]. This is believed to be due, at least in part, to the widespread and persistent use of various risk factors, the most common ones being areca nut and betel quid [18]. Local data also supports this as paan, supari and chalia were the most frequently used substances in our patient population, which are all various cultural combinations of betel quid and areca nut, sometimes including tobacco. The 5 year survival rate of OSCC ranges from 40-50% while the rate of recurrence is an alarming 50% within the first two years of diagnosis.
An interesting role of EGFR may be in encouraging premalignant lesions towards malignancy. A majority of males and an average age of 55 years was observed by Ries at al, in their premalignant population [21]. We report similar demographics with more male patients in our study and an average age of 45 years. Another study concerning only oral submucous fibrosis cases also on the Pakistani population reported a range of 18-54 years and a majority of males [37]. The study group of Benchekroun et al. assessed EGFR expression in 162 patients with oral premalignant lesions and found the female sex and a higher age to be significant factors for EGFR expression [22]. Moreover, our study showed that the upper/lower lip, as a site of lesion was an important factor in predicting EGFR overexpression. Carballeira et al. considered EGFR overexpression a bad prognostic criterion in lip squamous cell carcinoma patients as it had a marked effect on tumour ulceration and tumour thickness and width [38]. Although EGFR has been implicated in malignant transformation of premalignant lesions [22, 23] exactly how it is overexpressed in terms of the mechanism of action needs further elucidation. This may be possible due to direct exposure to the sun as has been suggested by Yardimici et al. [39].
The OSCC patient population characteristics of our study were in alignment with previous studies from our population which also report the cheek as the most common site of lesion and moderately differentiated AJCC stage II as most frequent diagnosis for our population and wider geographical area [40] [41]. A mean age of 52 years and an almost equal incidence of both genders was found in our study which corroborated previous reports [42] [6].
EGFR was overexpressed in the majority of our patients at 67%. Grandis et al. reported EGFR overexpression in 90% of HNSCC cases [43], while others report an average of 70-80% in oral and oropharnygeal cancers [44-46]. To the best of our knowledge, no studies regarding EGFR status in OSCC have been performed on our population, thus a parallel cannot be drawn. The Indian population can be considered geographically, genetically and habitually close to ours. EGFR positivity was reported in 27.5% and 72.5% of OSCC cases as low and high expression respectively, by Gupta et al. which corroborates our findings [33]. Another study concluded a median overexpression of 50% [46]. However, Khan et al reported an outstanding 92.8% positivity for EGFR which is well above our findings [47]. Considering site-specific expression, EGFR overexpression was seen in cheek (46%) more than the tongue (24%). This may be correlated with the chewing habits of our population since it is a common practice to stuff paan/supari between the gingiva and cheek which allows maximum penetration and absorption, and this was also the most commonly used substance of our data set.
The majority of people who were involved in at least one kind of habit were confirmed EGFR positive (67%) and this was statistically significant (p=0.039), which leads to the possibility that these substances maybe acting on the EGFR gene and causing mutations which result in overexpression of the receptor. This has been demonstrated by previous studies in which EGFR was identified as one of the most commonly mutated genes due to exposure to risk factors in OSCC [19]. Previous accounts on our population have reported associations between oral cavity cancer incidences and the use of these, although EGFR has not been implicated as an agent of carcinogenesis before [12] [18].
Patient 5 year survival is the gold standard by which the viability of any prognostic biomarker is evaluated. The majority of our patients were living (57%) at the end of follow up and recurrence was observed in 65% of the total. Authors also report a 5-year survival rate of 40-50% [48] with 50% of patients developing recurrence within the first two years of treatment [4]. We determined that EGFR significantly and independently affected 5 year survival and recurrence, with EGFR positive patients having markedly lower survival rates and increased chances of recurrence.
Overall survival and recurrence at 5 years (univariate analysis)
| Factor | Group | 5 year survival | Disease Free Survival | ||||
|---|---|---|---|---|---|---|---|
| N | Months | P-value | N | Months | P-value | ||
| Gender | Male | 56 | 42.37 | 0.719 | 56 | 62.85 | 0.59 |
| Female | 44 | 42.81 | 44 | 88.94 | |||
| Age Division | < 40 years | 16 | 40.25 | 0.609 | 16 | 67.15 | 0.95 |
| ≥40 years | 84 | 43.01 | 84 | 81.39 | |||
| Histological Classification | Well Differentiated | 41 | 46.41 | 0.136 | 41 | 112.02 | 0.14 |
| Moderately Differentiated | 56 | 40.50 | 56 | 52.64 | |||
| Poorly Differentiated | 3 | 28.66 | 3 | 41.33 | |||
| AJCC Stage | I | 18 | 49.66 | 0.004* | 18 | 72.47 | 0.01* |
| II | 30 | 49.70 | 30 | 123.63 | |||
| III | 27 | 42.11 | 27 | 47.32 | |||
| IV | 25 | 29.40 | 25 | 40.75 | |||
| Habits | Yes | 73 | 42.98 | 0.879 | 73 | 63.34 | 0.26 |
| No | 19 | 40.10 | 19 | 75.27 | |||
| Unknown | 8 | 44.62 | 8 | 104.50 | |||
| Primary site | Cheek | 65 | 41.04 | 0.338 | 65 | 62.28 | 0.38 |
| Tongue | 35 | 45.40 | 35 | 93.99 | |||
| Primary margins | Clear | 62 | 45.27 | 0.187 | 62 | 81.75 | 0.85 |
| Near | 30 | 40.46 | 30 | 61.68 | |||
| Involved | 8 | 29.50 | 8 | 59.56 | |||
| Final T stage | T1 | 21 | 49.52 | 0.075 | 21 | 65.47 | 0.258 |
| T2 | 44 | 43.72 | 44 | 101.94 | |||
| T3 | 16 | 43.93 | 16 | 50.64 | |||
| T4 | 19 | 31.05 | 19 | 44.42 | |||
| Final N stage | N0 | 75 | 47.40 | <0.001* | 75 | 98.52 | <0.001* |
| N1 | 17 | 30.88 | 17 | 28.04 | |||
| N2 | 8 | 22.12 | 8 | 11.25 | |||
| Radiotherapy | Yes | 58 | 39.82 | 0.223 | 58 | 51.14 | 0.16 |
| No | 32 | 46.25 | 32 | 98.52 | |||
| Unknown | 10 | 46.70 | 10 | 93.06 | |||
| EGFR expression | Positive | 67 | 39.28 | 0.048* | 67 | 56.82 | 0.01* |
| Negative | 33 | 49.24 | 33 | 99.10 | |||
| EGFR staining intensity | Negative | 33 | 49.24 | 0.015* | 33 | 99.10 | 0.01* |
| Mild | 11 | 47.81 | 11 | 82.03 | |||
| Moderate | 41 | 34.41 | 41 | 41.42 | |||
| Strong | 15 | 46.33 | 15 | 44.73 | |||
Overall survival and recurrence at 5 years (multivariate analysis)
| Factor | Group | 5 year survival | Disease free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Exp (β) | P - value | 95% CI of Exp (β) | N | Exp (β) | P - value | 95% CI of Exp (β | ||
| Final N stage | N0 | ** | ** | ** | ** | 75 | 1 | <0.001* | |
| N1 | 17 | 2.373 | 1.27-4.41 | ||||||
| N2 | 8 | 5.382 | 2.28-12.66 | ||||||
| EGFR expression | Positive | 67 | 2.024 | 0.056* | 0.983-4.165 | 67 | 2.39 | 0.004* | 1.32-4.31 |
| Negative | 33 | 1.00 | 33 | 1.00 | |||||
| AJCC Stage | I | 18 | 0.003* | † | † | † | † | ||
| II | 30 | 0.977 | 0.327-2.92 | ||||||
| III | 27 | 1.606 | 0.564-4.573 | ||||||
| IV | 25 | 3.705 | 1.359-10.09 | ||||||
*p-value considered significant at <0.05
**Final N stage was not significant for living status
†AJCC stage was not significant for recurrence
This provides compelling evidence for the use of EGFR as a prognostic tool since it was as strong a predictor of survival and recurrence as AJCC staging and nodal metastasis. In accordance with our study, Gupta et. al also showed that EGFR overexpression was significantly related to recurrence and added that it also meant an early time to recurrence [4]. Moreover, an improved disease-free interval has been observed with low levels of EGFR accompanied by chemoradiation [33]. Another study using tissue microarray technology also concluded that EGFR overexpression (cytoplasmic and membranous both) had a negative prognostic influence as overall and disease-free survival both were lowered [49].
The use of EGFR in targeted anticancer therapy is showing differing yet promising results in OSCC. A study based on functional genomics has clarified that certain mutations are more responsive towards targeted therapy, highlighting the importance of knowing the exact mutations in our patients before we begin their therapy [50].
Regarding premalignant lesion transition to malignancy, our data could not conclude a significant role for EGFR, possibly due to the low sample size and technique involved. For OSCC however, EGFR was proved a reliable biomarker for predicting the overall survival and disease free survival of patients, with as much of an independent impact as AJCC staging and lymph node involvement, and it may be used to identify high-risk subgroups in our population and guide their subsequent therapy.
Abbreviations
OSCC: oral squamous cell carcinoma; EGFR: epidermal growth factor receptor; AKUH: Aga Khan University Hospital; HNSCC: head and neck squamous cell carcinoma; AJCC: American Joint Commission on Cancer; TNM: tumour-nodes-metastases.
Acknowledgements
This study was funded by a grant from Higher Education Commission, Pakistan awarded to Dr Syed Muhammad Adnan Ali. Award Number: PM PFP/HRD/HEC/2012/4015.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309-16
2. Stewart B, Wild CP. World cancer report 2014. 2014.
3. Martinez-Useros J, Garcia-Foncillas J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015;51:423-30
4. Gupta S, Kushwaha VS, Verma S, Khan H, Bhatt M, Husain N. et al. Understanding molecular markers in recurrent oral squamous cell carcinoma treated with chemoradiation. Heliyon. 2016;2:e00206
5. Jadhav KB, Gupta N. Clinicopathological prognostic implicators of oral squamous cell carcinoma: need to understand and revise. N Am J Med Sci. 2013;5:671
6. Bhurgri Y, Bhurgri A, Usman A, Pervez S, Kayani N, Bashir I. et al. Epidemiological review of head and neck cancers in Karachi. Asian Pac J Cancer Prev. 2006;7:195-200
7. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
8. PMRC. Malignant Tumours. Report of a multicentric study PMRC. 1982
9. Parkin DM, Arslan A, Bieber A, Bouvy O, Muir C, Owor R. et al. Cancer occurrence in developing countries: IARC Lyon; 1986.
10. Bhurgri Y. Cancer of the oral cavity - trends in Karachi South (1995-2002). Asian Pac J Cancer Prev. 2005;6:22-6
11. Khawaja MI, Shafiq M, Nusrat R, Khawaja MR. Preventing the oral cavity cancer epidemic. Asian Pac J Cancer Prev. 2005;6:420
12. Alamgir M. Chewable Tobacco Consumption-A menace of oral cancer in Karachi. Pakistan Journal of Medicine and Dentistry. 2014;3:1-2
13. Organization WH. IARC Monographs Programme finds betel-quid and areca nut chewing carcinogenic to humans. Geneva. 2003
14. Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP. et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777-89
15. Travasso C. Betel quid chewing is responsible for half of oral cancer cases in India, finds study. BMJ. 2013;347:f7536
16. Ranganathan K, Rooban T, Rao U. Oral squamous cell carcinoma in patients with and without predisposing habits in glossal and extra-glossal site: An institutional experience in South India. Indian J Cancer. 2015;52:625
17. Kerishnan JP, Gopinath SC, Kai SB, Tang T-H, Ng HL-C, Rahman ZAA. et al. Detection of Human Papillomavirus 16-Specific IgG and IgM Antibodies in Patient Sera: A Potential Indicator of Oral Squamous Cell Carcinoma Risk Factor. International journal of medical sciences. 2016;13:424
18. Bhurgri Y, Bhurgri A, Hussainy AS, Usman A, Faridi N, Malik J. et al. Cancer of the oral cavity and pharynx in Karachi-identification of potential risk factors. Asian Pac J Cancer Prev. 2003;4:125-30
19. Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884
20. Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. International Journal of Radiation Oncology* Biology* Physics. 2004;58:903-13
21. Ries J, Vairaktaris E, Agaimy A, Bechtold M, Gorecki P, Neukam FW. et al. The relevance of EGFR overexpression for the prediction of the malignant transformation of oral leukoplakia. Oncol Rep. 2013;30:1149-56
22. Benchekroun MT, Saintigny P, Thomas SM, El-Naggar AK, Papadimitrakopoulou V, Ren H. et al. Epidermal growth factor receptor expression and gene copy number in the risk of oral cancer. Cancer Prev Res. 2010;3:800-9
23. Poh C, Zhu Y, Chen E, Berean K, Wu L, Zhang L. et al. Unique FISH patterns associated with cancer progression of oral dysplasia. Journal of dental research. 2012;91:52-7
24. Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer research. 2002;62:7350-6
25. Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS journal. 2013;280:5350-70
26. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S. et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123-32
27. Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, Liu DD. et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. Journal of Clinical Oncology. 2007;25:2164-70
28. Alanazi IO, Khan Z. Understanding EGFR signaling in breast cancer and breast cancer stem cells: overexpression and therapeutic implications. Asian Pac J Cancer Prev. 2016;17:445-53
29. Yiu AJ, Yiu CY. Biomarkers in colorectal cancer. Anticancer research. 2016;36:1093-102
30. Matthews LM, Noble F, Tod J, Jaynes E, Harris S, Primrose J. et al. Systematic review and meta-analysis of immunohistochemical prognostic biomarkers in resected oesophageal adenocarcinoma. Br J Cancer. 2015;113:107-18
31. Nakagaki T, Sasaki Y, Idogawa M, Koyama R, Kobashi K, Tamura M. et al. Semiconductor-based next-generation sequencing analysis of 409 cancer-related genes for mutations and copy-number variations in oral squamous cell carcinoma. AACR. 2016
32. Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E. et al. Tumor microenvironment in head and neck squamous cell carcinoma. Seminars in oncology: Elsevier. 2014 p. 217-34
33. Gupta S, Khan H, Kushwaha VS, Husain N, Negi M, Ghatak A. et al. Impact of EGFR and p53 expressions on survival and quality of life in locally advanced oral squamous cell carcinoma patients treated with chemoradiation. Cancer Biol Ther. 2015;16:1269-80
34. Keren S, Shoude Z, Lu Z, Beibei Y. Role of EGFR as a prognostic factor for survival in head and neck cancer: a meta-analysis. Tumour Biol. 2014;35:2285-95
35. Troiani T, Napolitano S, Della Corte CM, Martini G, Martinelli E, Morgillo F. et al. Therapeutic value of EGFR inhibition in CRC and NSCLC: 15 years of clinical evidence. ESMO open. 2016;1:e000088
36. Bhurgri Y, Bhurgri A, Hassan SH, Zaidi S, Rahim A, Sankaranarayanan R. et al. Cancer incidence in Karachi, Pakistan: first results from Karachi cancer registry. International journal of cancer. 2000;85:325-9
37. Razi A, Iqbal A, Ali H, Kashif M. Oral Submucous Fibrosis: Successful Management of Fifty Cases with Interpositioning Buccal Fat Pad Flap. Annals of Abbasi Shaheed Hospital & Karachi Medical & Dental College. 2016:21
38. Carballeira A, Ginarte M, Diniz-Freitas M, Fernández-Campos I, Gude F, Fraga M. et al. Immunohistochemical evaluation of EGFR expression in lip squamous cell carcinoma. Correlation with clinicopathological characteristics. Histol Histopathol. 2014;29:641-8
39. Yardimci G, Kutlubay Z, Engin B, Tuzun Y. Precancerous lesions of oral mucosa. World Journal of Clinical Cases: WJCC. 2014;2:866
40. Alamgir M, Jamal Q, Jafarey N, Mirza T. Clinicopathological parameters of 50 oral squamous cell carcinoma cases in Karachi. Pakistan Journal of Medicine and Dentistry. 2013;2:3-8
41. Alamgir MM, Jamal Q, Mirza T. Conventional clinical and prognostic variables in 150 oral squamous cell carcinoma cases from the indigenous population of Karachi. Pak J Med Sci. 2016;32:672
42. Bhurgri Y, Rahim A, Bhutto K, Bhurgri A, Pinjani P, Usman A. et al. Incidence of carcinoma of the oral cavity in Karachi-District South. J Pak Med Assoc. 1998;48:321-4
43. Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer research. 1993;53:3579-84
44. Sarkis SA, Abdullah BH, Majeed BAA, Talabani NG. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol. 2010;2:13
45. Laimer K, Spizzo G, Gastl G, Obrist P, Brunhuber T, Fong D. et al. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: a TMA-based immunohistochemical analysis. Oral Oncol. 2007;43:193-8
46. Won HS, Jung C-K, Chun SH, Kang J-H, Kim Y-S, Sun D-I. et al. Difference in expression of EGFR, pAkt, and PTEN between oropharyngeal and oral cavity squamous cell carcinoma. Oral Oncol. 2012;48:985-90
47. Khan H, Gupta S, Husain N, Misra S, Negi M, Jamal N. et al. Correlation between expressions of Cyclin-D1, EGFR and p53 with chemoradiation response in patients of locally advanced oral squamous cell carcinoma. BBA clinical. 2015;3:11-7
48. Martinez-Useros J, Garcia-Foncillas J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015;51:423-30
49. Monteiro LS, Diniz-Freitas M, Garcia-Caballero T, Warnakulasuriya S, Forteza J, Fraga M. Combined cytoplasmic and membranous EGFR and p53 overexpression is a poor prognostic marker in early stage oral squamous cell carcinoma. J Oral Pathol Med. 2012;41:559-67
50. Sheu JJ-C, Hua C-H, Wan L, Lin Y-J, Lai M-T, Tseng H-C. et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer research. 2009;69:2568-76
Author contact
![]() Corresponding author: Syed Muhammad Adnan Ali, Ph.D (Clinical Pathology), Assistant Professor, Department of Surgery, Aga Khan University Hospital, Stadium Road P.O. Box 3500, Karachi 74800, Pakistan. Email: syed.adnanedu; Phone: 92-21-34930051 (Ext 4748); Fax: (92 21) 3493-4294 & 3493-2095
Corresponding author: Syed Muhammad Adnan Ali, Ph.D (Clinical Pathology), Assistant Professor, Department of Surgery, Aga Khan University Hospital, Stadium Road P.O. Box 3500, Karachi 74800, Pakistan. Email: syed.adnanedu; Phone: 92-21-34930051 (Ext 4748); Fax: (92 21) 3493-4294 & 3493-2095