Oncomedicine 2018; 3:37-47. doi:10.7150/oncm.25566 This volume Cite
Review
The Role of Melatonin in Cancer Development
1. Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 38117. USA.
2. Departments of Biology and Advanced Placement Biology, White Station High School, Memphis, Tennessee 38117. USA.
3. Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Memphis, TN 38163. USA.
Received 2018-2-14; Accepted 2018-4-26; Published 2018-5-27
Abstract
Melatonin is a hormone that is secreted by the pineal gland in the brain. Its secretion during periods of darkness represents a 24-hour rhythm controlled by the circadian clock and is pivotal in the regulation of sleep and wake cycles. Although circadian rhythms are endogenous processes, they can be affected by environmental factors such as light and temperature. In addition to its role in circadian rhythms, melatonin has also been observed to function in the immune system, female menstrual cycles, seasonal behavior, tumor inhibition, and anti-aging processes. [1] Melatonin production increases during the night and decreases in the daytime with exposure to light. Low levels of normal melatonin have been linked to neoplasia, such as the growth of rat hepatomas and human breast cancer xenografts, circadian phase shifts, sleep disturbances, and immunosuppression. [3,4] Furthermore, sleep disturbance, not factoring in melatonin production, can lead to immune suppression and a shift to the predominance in cancer-stimulatory cytokines. [4] To combat cancer, studies have shown that melatonin significantly suppresses cell proliferation and induces apoptosis. [2] There have been no direct links determined between abnormal melatonin production observed in sleep disturbances and cancer, so this review will focus on the experimental evidence depicting the role of melatonin in the prevention of cancer development.
Keywords: Melatonin, cancer development
Introduction
Since the discovery of melatonin in the early 1900s, significant advancements have provided researchers with the technology to study the hormone in-depth, determine the myriad of processes it acts in, and develop treatment methods for various diseases. By the late 1900s, melatonin's antioxidant property was discovered, and later in the 2000s, studies conducted have affirmed its anticancer effects. [6] In addition to its role in circadian rhythms and the maintenance of regular sleep patterns, its effects have been studied in cancer development and cancer treatments.
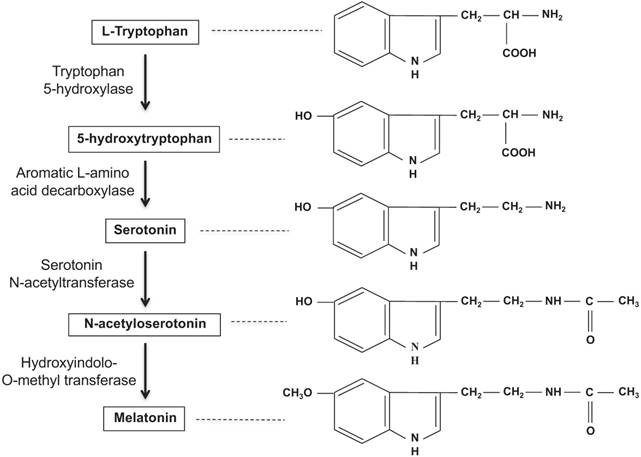
The pineal gland hormone, melatonin, is also known as 5-methoxy-N-acetyltryptamine and is synthesized from the amino acid tryptophan and converted into serotonin. [5] Acetylation of the serotonin through the enzyme arylalkylamine N- acetyltransferase (AANAT) functions to form N-acetylserotonin. N-acetylserotonin is then finally converted into melatonin by the enzyme hydroxyindole-O-methyltransferase (Figure 1). [5] As a hormone that readily crosses the blood-brain barrier (BBB), melatonin plays a protective role against oxidation disorders of cerebral nerve cells, detoxifies carcinogens by activating glutathione and anti-oxidative pathways, and protects or repairs cellular DNA damage. [5] Melatonin production is heavily intertwined with the biological clock and thus the 24-hour human circadian rhythm and sleep patterns. During the night, melatonin production peaks and then falls dramatically during the day with exposure to light. Although the occurrence of sleep is not necessary for the nocturnal production of melatonin, the presence of darkness is a requirement. As long as an individual is exposed to total darkness, the circadian melatonin signal should remain intact. [4] Increased melatonin secretion typically corresponds with an increase in sleep propensity and a decrease in core body temperature; the opposite occurs with reduced levels of melatonin secretion. [4] Although circadian rhythms are internal, abnormal sleep patterns and exposure to light can induce phase shifts in the biological rhythm. [8]
Melatonin biosynthesis in the pineal gland. Adapted from Ref. [60]
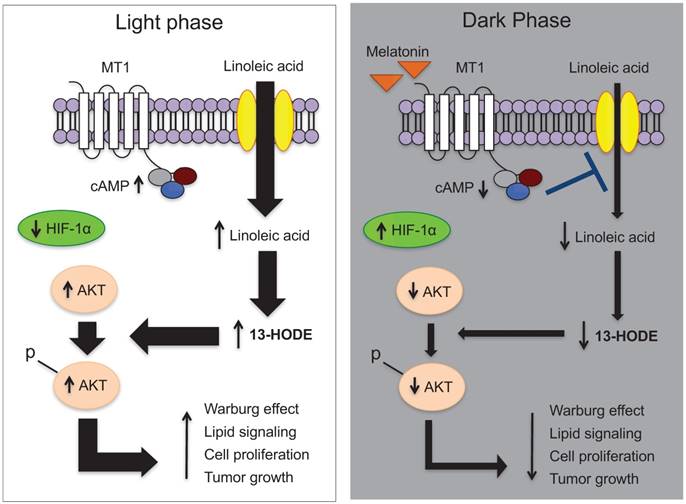
The effectiveness of melatonin in suppressing various illnesses and cancers has been proven through multiple in vitro and in vivo experiments. Apoptosis is the primary mechanism by which melatonin converts tumor cells to healthy cells. [5] Higher levels of melatonin suppress TP53 (the tumor-promoting gene), COX-2, p300, and NF-kB signaling, and they inhibit the uptake of linoleic acid (LA), which is responsible for fatty acid growth and tumor cell proliferation. [2, 3, 5] Furthermore, dietary supplementation with melatonin has been shown to suppress cAMP formation, and by inhibiting tumor uptake of LA and its metabolism to 13-HODE, the growth of rat hepatomas was inhibited. [3] Melatonin also inhibits cell proliferation and induces apoptosis in MDA-MB-361 breast cancer cells in vitro by simultaneously suppressing the COX-2/PGE2, p300/NF-κB, and PI3K/Akt/signaling and activating the Apaf-1/caspase-dependent apoptotic pathway. [2] Evidence has shown that administration of melatonin alone or in combination with interleukin-2, chemoradiotherapy, and supportive care in late-stage tumors can lead to improved outcomes of tumor regression and overall individual survival. [7] These methods in conjunction with the increasing amount of evidence supplied through various melatonin studies give scientists the potential to create new preventive/therapeutic strategies to optimize host/cancer balance for host survival and quality of life. [3]
Role of melatonin in circadian rhythms
Melatonin is a hormone secreted by the pineal gland, and its secretion follows a nocturnal circadian rhythm dependent upon the biological clock, an animal's internal 24-hour cycle. The central clock in the body is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, and it maintains temporal homeostasis by delivering messages throughout the body about the time of day. [27] The pineal gland also supports an internal clock using melatonin release. The clock-setting property of melatonin suggests its role as a “chronobiotic” that alters and normalizes biological rhythms. [31] As a result, cellular processes can synchronize and progress at normal times to ensure the stability of an organism and its activities.
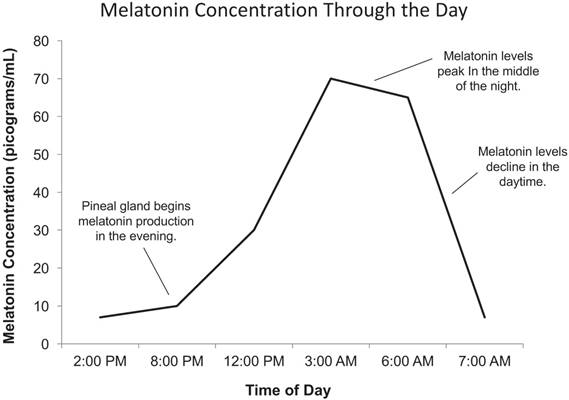
Levels of melatonin (MEL) are regulated by various mechanisms including norepinephrine, the clock, and the presence of light. Production of melatonin is stimulated by the presence of norepinephrine, which is released from sympathetic nerve fibers exclusively at night. [10] In the absence of noradrenergic stimulation during the day, melatonin is immediately degraded through proteasomal proteolysis. [10] The primary physiological function of melatonin is to convey information concerning the daily cycle of light and darkness to body physiology in response to changes in photoperiods as well as to organize other circadian rhythms and day length dependent changes, such as reproductive competence. [9, 17] Studies of seasonal breeding hamsters and sheep indicate that circadian clock gene expression in the pars tuberalis (PT) of the pituitary gland is modulated by photoperiods via melatonin signals. [18] The amount of melatonin typically decreases in the daytime with exposure to light and increases during the night, reaching its peak around 3:00 AM (Figure 2). The close relationship between the time of day and the production of melatonin suggests its importance in the promotion of sleep. Although sleep is not necessary for the production of melatonin, the presence of darkness is an absolute requirement. [4] Even when individuals are exposed to lengthy periods of total darkness, the biological clock, and thus the rhythm of melatonin secretion, remains relatively stable.
Daily cycle of melatonin production in humans. Adapted from Ref. [5]
Environmental effects on melatonin production
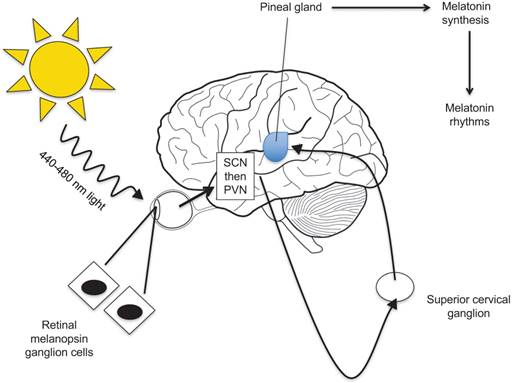
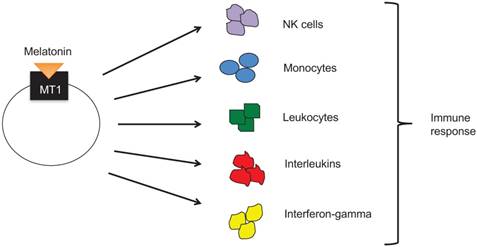
Melatonin secretion and release display a circadian rhythm, reaching its highest levels during the night and early morning hours when there is minimal light and its lowest levels during a period of light. Pineal glands of lower vertebrates are simulated directly by light. Higher level vertebrates, including humans, however, have pineal glands that are not directly photosensitive. Rather, the gland responds to light via a multisynaptic pathway that includes retinal ganglion cells and the photopigment melanopsin. [33]
The retina, which contains three types of photoreceptors (rods, cones, and ganglion cells), receives information about light and dark. Ganglion cell axons are then used to transmit light signals to certain brain centers for “nonimage” visual functions such as circadian photoentrainment. [28] From the retina, sodium-dependent action potentials are required for the light signal to travel to the pineal gland to regulate melatonin levels in the body (Figure 4). [29]
Several studies have shown that melatonin is produced not only in the pineal gland but also in ocular tissues, specifically the retina, ciliary epithelial cells, and lens cells. [34] Furthermore, the rhythms that control melatonin synthesis and release in the retina are entrained by light, are temperature compensated, and have been shown to persist both in vivo and in vitro. [35] Melatonin also appears to entrain the biological clock via activation of G-protein coupled integral membrane melatonin receptors, which have been identified in several ocular tissues, such as the neural retina, ciliary body, cornea, and lens. [34]
In humans, the action spectra for melatonin suppression has a maximum around 460 nm suggesting that melanopsin, a functional photopigment, plays a role in the photic regulation of melatonin levels, circadian rhythms, and sleep patterns. [29] Further data shows that blue light with wavelengths between 440-480 nm is highly effective in phase-shifting the human circadian clock and suppressing melatonin secretion. [30] These results provide support for avoiding blue light before sleeping to maximize melatonin secretion. Given that light affects melatonin secretion and melatonin secretion in turn correlates to sleep, external forms of melatonin has been used to treat insomnia, jet lag, and other sleep disorders.
Correlation between melatonin levels and development of sleep disorders
The various roles of endogenous melatonin secretion in cellular and physiological processes have furthered its use as an exogenous medication for sleep disorders. Studies have shown a correlation between melatonin levels and sleep. Nocturnal melatonin levels and the quality of sleep tend to decline past puberty. [31]
Traveling between different time zones results in an experience commonly known as jet lag, which is characterized by sleep disturbances and fatigue. These side effects occur due to the inability of the circadian clock to rapidly synchronize to the new environment. As a result, melatonin (with its apparent role in clock-setting) has been used to combat the effects of jet lag. When the drug is taken at the destination between 10 pm and midnight, it can correct the side effects of jet lag. [31] However, abnormal timing of melatonin ingestion can worsen symptoms. A trial conducted on an international cabin crew showed that use of melatonin before arrival had negative effects on recovery while the use of melatonin upon arrival at the destination prompted a significantly faster recovery of energy and alertness. [36]
Older people and insomniacs typically display reduced nocturnal melatonin levels and thus are more prone to sleep disturbances. Melatonin has been used to alleviate the effects of insomnia and reinstate regular nocturnal melatonin levels; however, other studies have shown minimal correlation between the use of melatonin supplements and improved sleep. In several small studies, 5-mg doses of melatonin given at 10 pm resulted in the advancement of sleep by 1.5 hours and reduced sleep duration by 30 minutes. [31] A study conducted on melatonin treatment for age-related insomnia showed that the daytime administration of melatonin to young people in doses that mimic nocturnal levels could accelerate sleep onset. In contrast, insomniacs who were given melatonin had little change in their sleep. [37]
Melatonin has also been found to have positive effects in treating narcolepsy, a chronic sleep disorder characterized by sudden attacks of sleep, overwhelming drowsiness, and rapid-eye-movement sleep deficit. [27, 31] In a series of clinical trials involving patients with rapid eye movement sleep behavior disorder (RBD), the majority of patients experienced persistent benefits from melatonin after one year of therapy. [39] One study administered melatonin and arginine vasotocin (AVT) to three male narcoleptics, and the results showed an increased amount of REM sleep and a decreased REM sleep latency. [40] Further reports indicated an increase in dream activity after administration of extremely high doses of melatonin.
Studies conducted by Buscemi tested the effectiveness of exogenous melatonin in normal sleepers and those with sleep disorders. It was discovered that in normal sleepers, melatonin had an insignificant effect on REM latency. However, melatonin was found to decrease sleep onset latency significantly in those with primary sleep disorders. [63] Primary sleep orders are classified as those not attributable to a medical or psychiatric condition, such as insomnia, narcolepsy, and sleep apnea. [64] Secondary sleep disorders are those that occur as a result of a primary sleep disorder. [65] Moreover, sleep onset latency (SOL) was decreased in insomnia patients. Overall, the studies showed that exogenous melatonin had an effect on SOL in patients with primary sleep disorders but not on sleep efficiency. The findings were reverse in people with secondary sleep disorders. [63].
Mounting data shows benefits of using melatonin to promote and improve sleep. As a result, melatonin supplementation has been used in autistic children who display abnormal melatonin circadian rhythms and low levels of melatonin. [41] Melatonin administration in ASD patients has been associated with improved sleep, better daytime behavior, and minimal side effects. [41] Further research must be conducted on the efficacy of melatonin to reduce the effects of different sleep and behavioral disorders, but current data suggests a widening potential of the drug as a successful therapy.
Melatonin acts as an antioxidant and free-radical scavenger
In addition to its role in sleep promotion and circadian clocks, melatonin also serves as a protector of macromolecules, nuclear DNA, and mitochondrial DNA by acting as a potent antioxidant and free-radical scavenger. Melatonin directly scavenges free radicals, stimulates various antioxidant enzymes, inhibits oxidative enzymes, increases the efficiency of mitochondrial oxidative phosphorylation, and thereby reduces electron leakage and the generation of free radicals. [11,13] Moreover, it has been shown that melatonin also stabilizes cellular membranes, aiding in the resistance of oxidative and cellular damage. [11]
Functions of melatonin in the immune system
Not only does melatonin act as an antioxidant and free-radical scavenger, but it also plays a role in the immune system. An early indication of the innate immune response is the proliferation of cytokines, including TNF-a, IL-1B, and IL-6 by activated macrophages, all of which are characteristic of the inflammatory response. Studies have shown that splenocytes from healthy mice treated with high doses of melatonin produced increased levels of IL-1B. [13] Mice that were subjugated to trauma-hemorrhage cycles displayed depressed immune functions; however, after administered doses of melatonin, these mice had a significantly improved immune system shown by the restoration of peritoneal macrophages (Mphi) as well as IL-1, IL-2, IL-3, and IL-6 release. [15] MEL has also been reported to enhance the production of several interleukins, including IL-6, IL-1, and IL-12 in human monocytes. [14] Further studies conducted on human monocytes suggest that melatonin doses above a given threshold, 10 (-12) M, induce the activity of IL-1 alpha as well as IL-1 beta. Below this threshold melatonin does not induce activity for IL-1 but rather causes the translation of IL-1 mRNA. [16] T-helper cells express G-protein coupled cell membrane MEL receptors. Activation of these receptors enhances the release of T-helper cells Type 1 (Th1) cytokines, such as gamma-interferon and IL-2. [14] The various cytokines released may counteract stress-induced immunodepression and other secondary immunodeficiencies. MEL also is involved in the generation of blood cells and platelets in the bone marrow through the stimulation of hemopoietic cytokines or directly affecting progenitor cells such as pre-B cells, monocytes, and natural killer cells. The hemopoietic effect of MEL in mice and cancer patients are associated with diminished toxicity of chemotherapeutic treatments, thus suggesting a potential addition to the regimen of chemotherapy patients in the effort to reduce the adverse side effects of the treatment. [14]
Overview of the role of melatonin in cancer development
Melatonin has been known to affect the growth of tumors, and its secretion corresponds with the presence of light and a circadian rhythm. The relationship between melatonin secretion, light, and cancer suggests that an increase in the prevalence of various types of cancer corresponds with industrialization and exposure to more forms of artificial light, thus resulting in lower levels of nocturnal melatonin. In animals, pineal suppression and pinealectomies stimulate the growth and metastasis of experimental cancers of the lung, liver, ovary, pituitary, and prostate. [31] Mouse studies using different melatonin administration protocols showed that the drug decreased the incidence and size of mammary adenocarcinomas and lung metastases. [44]
Clinical evidence and research have also shown the prospective use of melatonin as a treatment for breast cancer patients. It was found that in women with breast cancer, the melatonin levels both in morning and evening were abnormal. Nocturnal melatonin levels were low while morning urine samples of breast cancer patients displayed high MEL levels, the opposite of what is expected in healthy individuals. [42] Melatonin can bind to several receptors including MT1 and MT2, which are located in lymphocytes, platelets, prostatic cells, renal tubules, and cardiac myocytes and can also bind to nuclear receptors. [5] Binding to nuclear receptors allows melatonin to alter the transcription of genes and inhibit cell proliferation. [5] Pre-treatments with melatonin in estrogen receptor-alpha (ERalpha-positive) MCF-7 human breast cancer cells reduced ERalpha transactivation and binding activity and decreased the elevation of cAMP levels. [43] Decreased cAMP levels suppress the uptake and metabolism of linoleic acid (LA) and results in a reduction of the activation of the epidermal growth factor pathway and thus tumor growth inhibition. [4] In two studies conducted by Lissoni in 1995, melatonin was administered to breast cancer patients to observe the effects on tumor regression and other side effects. Women with metastatic breast cancer who had not responded to tamoxifen (TMX) alone were given TMX at noon and melatonin in the evening. Final reports showed a reduction of lesions and a decrease in insulin-like growth factor (IGF-1) in patients. [45] A second trial was conducted to evaluate the effects of high-dose melatonin in breast cancer patients receiving chemotherapy, which due to its high toxicity limited the frequency of treatment. After four cycles, the melatonin administered was observed to normalize platelet levels in a majority of the test subjects and cause tumor regression in 5 out of 12 patients with little toxicity reported. [45] The vast amount of data compiled concerning melatonin supplements in patients shows its use as a potentially widely sought anti-cancer therapy.
Melatonin has also been shown to improve survival rates in patients with prostate and colorectal cancer. Studies have shown that men with primary localized malignant prostate tumors have extremely low levels of nocturnal melatonin that decrease with an increase in tumor growth. [46] Patients with unoperated colorectal carcinoma were found to have a significantly lower nocturnal plasma melatonin level compared to controls. [47] However, a second study conducted by Kvetnaia et al. found a higher nocturnal urinary metabolite 6-sulfatoxymelatonin (aMT6s) excretion in operated, untreated males. In one study of 54 patients with metastatic lung and colorectal tumors, the melatonin regimen resulted in stabilization of cancer and improved quality of life for roughly 40% of the recipients. [31, 48] As specified previously, the effects of melatonin supplementations can change according to the time of administration. Melatonin injections given in the morning have been found to stimulate cancer growth while injections in the evening contribute to tumor regression. Afternoon injections have no apparent effect. [31]
The relative levels of nocturnal melatonin can be measured by observing the amount of urinary aMT6 excreted. In addition to cancers of the reproductive system, melatonin has also been shown to affect tumor growth in the thyroid, bronchi, and stomach among other forms of cancer. In female patients with thyroid cancer, the levels of aMT6s were extremely low and did not differ from patients with benign thyroid diseases, indicating that thyroid growth negatively affects the proper regulation of the pineal gland. [48] In contrast to this finding, Karasek et al. found significantly elevated levels of aMT6s in thyroid cancer patients. [46] Similarly, several found depressed nocturnal aMT6s-excretion in early stage male bronchial cancer patients while others reported high morning and nighttime melatonin levels of late-stage patients. [49, 50] Furthermore, male patients with primary, unoperated stomach cancer displayed a depletion of the nocturnal urinary excretion of aMT6s. Results from other melatonin-cancer studies achieved similar results: some studies reported reduced nocturnal aMT6s levels while other studies reported high levels. Results from various studies show that melatonin has an apparent effect on cancer development, but the discrepancies among the studies prompt further research into the relationship between melatonin and cancer.
Relationship between melatonin and cancer risk
With industrialized society drifting more towards a life situated in the presence of 24-hour light, the occurrence of sleep disorders and abnormal sleep patterns continues to increase. As a result, circadian rhythms regulating cellular and physiological processes, such as the secretion of melatonin, have taken a significant toll on the ability to synchronize to the environment. Extended periods of exposure to light further inhibit the production and secretion of melatonin from the pineal gland through proteasomal proteolysis.
Decreased endogenous secretion of melatonin has been shown through various clinical and mouse studies and has been linked to a higher risk of certain types of cancer. Recent epidemiological studies have shown that women working night shifts are at a greater risk of breast, endometrial, and colorectal cancer while male night shift workers are at a significantly increased risk of developing prostate cancer presumably due to their increased exposure to light at night. [4] Individuals with various tumor types exhibited depressed nocturnal melatonin concentrations or nocturnal excretion of the main metabolite, 6-sulfatoxymelatonin. [1] There has been a particularly close link between melatonin levels and the risk of breast cancer. Data collected from the women involved in epidemiological studies included urinary melatonin levels (measuring specifically levels of 6- sulfatoxymelatonin), sleep duration, and shift work. [19] The results from the many studies conducted throughout the world, however, displayed contradicting information. Breast cancer risk was found to be elevated with decreased urinary melatonin levels in the Nurses' Health Study II, but results from the UK Guernsey Cohort study showed very little significant association between levels of urinary melatonin and the risk of breast cancer. [22, 23] Breast cancer risk was also found to decrease significantly with increased sleep duration in Finnish women. [24] The increase in the use of electricity in the past couple centuries has prompted a sharp increase in night-shift work. Prolonged exposure to light has been proven to decrease nocturnal melatonin levels and thus promotes an increase in the risk for various types of cancer. A 2009 analysis concluded that there is a 40% increase in the relative risk of developing breast cancer in women working night shifts. [45]
Furthermore, melatonin has been shown to inhibit the overproduction of estradiol, which induces cell division of breast cells. [5] Although several melatonin-cancer studies have provided enough data to suggest a correlation between levels of melatonin and the risk of cancer, other studies have also found results contrary to this hypothesis. A study conducted by Pinheiro on women in the Nurses' Health Study showed a moderate trend in increased breast cancer risk with longer sleep duration. [25] An additional large-population case study discovered similar results. The data showed a 6% increase in risk for every extra hour of sleep and thus suggests that increasing sleep duration is associated with a higher breast cancer risk. [26]
Melatonin and the prevention of cancer growth
A study published in the Journal of Pineal Research showed that 643 cancer patients who were unresponsive to conventional therapies were given large doses of melatonin as an alternative treatment. After a year the mortality rate of these patients was reduced by 34%. [19] Cancer patients in controlled trials conducted by Wang et al. further showed that melatonin significantly improved complete and partial remission, was associated with a 1-year survival rate, and dramatically decreased radiochemotherapy-related side effects including thrombocytopenia, neurotoxicity, and fatigue. [20] Mouse studies have demonstrated the inhibition of the development of breast cancer models by melatonin as well as the growth of tumors in rodents with exposure to long periods of light via a melatonin-induced suppression of tumor linoleic acid uptake and its metabolism to the mitogenic signaling molecule 13-HODE. [3] Due to melatonin's ability to suppress cAMP formation, it inhibits the uptake of LA and its metabolism to 13-HODE by a MEL receptor-mediated mechanism found in rat hepatomas and human breast cancer xenografts (Figure 3). [3] Endothelin-1 (EDN1) is an angiogenic factor that promotes the growth of tumors in the blood vessels. To combat this factor, melatonin inhibits the synthesis of endothelin-1 to block the growth of cancer cells (Figure 5A). [5] There is evidence that the administration of melatonin alone or in combination with interleukin-2 or chemoradiotherapy in patients with advanced tumors is associated with tumor regression and improved survival rates. [7] Melatonin prevents the proliferation of cancer cells by reducing telomere length and telomerase activity, both of which are responsible for the development of unhealthy cells and high levels of the hormone, suppressing the tumor-promoting gene TP53. [5] Additional data from clinical studies further establishes that the use of melatonin has minor side effects, and thus it is a hormone growing in popularity for the development of cancer treatments.
Nocturnal melatonin regulation of circadian rhythms. Adapted from Ref. [38]
MEL has been observed to repress cell proliferation through apoptotic pathways. Melatonin induces Apaf-1 expression, triggers cytochrome C release, and stimulates caspase-3 and caspase-9 activities and cleavage (Figure 5B), all of which are mechanisms characteristic of apoptosis. [2] Furthermore, high doses of melatonin in human neuroblastoma cells resulted in the induction of apoptosis in these cancer cells. Treatment with 1 mm melatonin for six days induced cell death in 75% of the cells. [21] In conjunction with the immune system, melatonin works to inhibit cancer cell growth. By stimulating natural killer (NK) cells, monocytes, leukocytes, interleukins, and interferon-gamma as well as activating the cytokine system and cytotoxic activity (Figure 6), melatonin helps maintain immunity to cancer cells. [5]
Retinal-pineal pathway of light transmission. Adapted from Ref. [61]
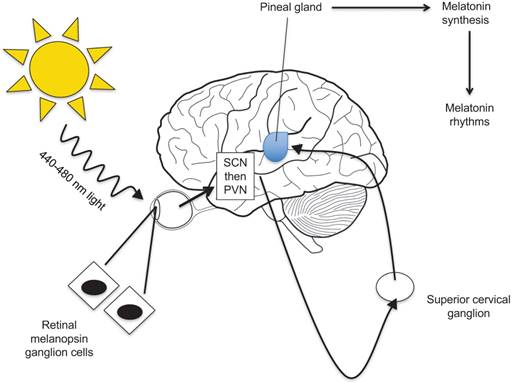
(A) Melatonin can suppress growth of tumors by inhibiting transcription of Endothelin-1 (EDN1), an angiogenic factor that promotes the formation of blood vessels around the tumor. (B) Melatonin can prevent cell proliferation through various systems, such as by blocking transcription of a gene determining cell growth or through cytochrome c release, caspase activity, and Apaf-1 expression. 
 means blocking.
means blocking.
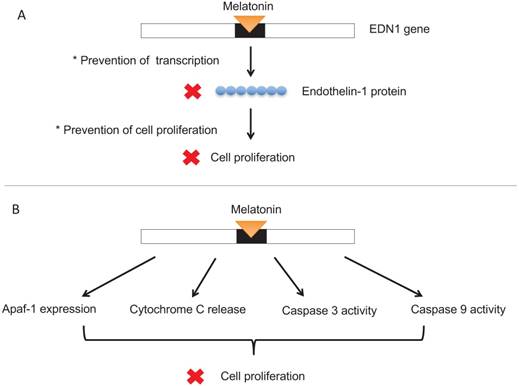
MEL stimulates cells of the immune system to invoke immune responses, defend the body from cancerous cells.
Adverse effects of melatonin as an anti-cancer treatment
Clinical trials using melatonin as an anti-cancer treatment and studies conducted on the role of melatonin in the prevention of cancer development show the surging potential of melatonin as a therapeutic medication to combat sleep disorders, cancer, and the side effects of chemotherapy. As seen in both animals and humans, melatonin has a very low level of toxicity, and even in relatively high doses, it typically does not cause major side effects. [31] Minor side effects, however, include headaches, dizziness, and drowsiness as well as nausea and apathy combined with weight gain. [32, 54] Melatonin treatment in children, however, can be sustained long-term without severe deviations of normal development, such as sleep quality, puberty, and mental health. [54]
Melatonin administration has been observed to change processes involved in male and female reproductive systems. In a 6-month double-blind study, eight healthy men were either given daily doses of melatonin or placebo. At the end of the trial, sperm count in the majority of the men was abnormal, and testosterone levels were elevated suggesting that long-term melatonin administration decreased semen quality. [51] Additional studies demonstrate that melatonin has dose-dependent negative effects on the forward progression of and motility of sperm in Wistar rats. [52] Melatonin has been shown to influence reproductive functions, but its precise role is still debated. MEL is the primary messenger of light-dependent periodicity and is implicated in seasonal reproduction in animals and puberty in humans. [55] Studies have shown that patients with hypothalamic hypogonadism have unusually high plasma melatonin concentrations, and some boys with delayed puberty have elevated daytime plasma melatonin levels. Over half of children with early sexual developments have lower levels of plasma melatonin concentrations. [53] Melatonin has also proved to be an effective female contraceptive with few side effects. [31] The hypothalamic GnRH pulse generator is activated and inactivated at different seasons largely based on the amount and duration of light available. The longer duration of the nighttime release of melatonin from summer to winter acts as an endocrine signal for inactivating the GnRH pulse generator, which is responsible for the activation of the pituitary-gonadal reproductive axis. [56] Through these mechanisms, melatonin can serve to block the pulse generator and act as a contraceptive.
Although melatonin has been successfully used in cancer and sleep disorder treatments, improper timing of use can result in negative outcomes. Melatonin injections in the morning can stimulate tumor growth; doses in the afternoon exhibit no effect, and doses in the evening have retarding effects. [31] Animal studies have shown that large doses of melatonin increased light-induced damage to retinal photoreceptors (ganglion cells, rods, and cones). [57] Furthermore, administration of melatonin that elongates the normal nocturnal melatonin pattern has been shown to exacerbate SAD, bipolarity, and classic depression. [31]
Melatonin is an easily accessible drug provided as an over-the-counter supplement. With its various roles in physiology and cellular processes, it also can interact with many other drugs, such as anticoagulants, interleukin-2, and antidepressant medications. [57] Studies show that melatonin can increase the risk of bleeding from anticoagulant medications such as warfarin, increase tumor regression and survival rates of cancer in conjunction with IL-2, and reduce antidepressant effects of drugs such as desipramine. [58, 59]
Conclusion
The pineal hormone and drug supplement, melatonin, plays a major role in maintaining circadian rhythms throughout the body. Not only is it involved in the normalizing of biological rhythms, but it is also observed to function in the immune system, in the reproductive system, as an antioxidant, and as an anti-cancer treatment. Secretion of melatonin itself follows a rhythm, reaching a peak during the night and early morning and decreasing with exposure to light, implicating that light and the quality of sleep are loosely, if not directly, correlated. To regulate the processes and rhythms of the body, melatonin is involved in a highly intricate pathway involving photoreceptors of the retina, which catch light and transmit the information through nerve cells, melatonin receptors, and the pineal gland. Studies have shown a higher risk for cancer development in people who have abnormal circadian rhythms, especially in those who are night-shift workers. With a greater exposure to artificial light, night shift workers display significantly lower nocturnal melatonin levels than their day shift counterparts. Through a series of apoptosis pathways and interactions with the immune system, chemotherapy, and anti-cancer drugs, melatonin can help increase tumor regression in cancer patients. Melatonin has been shown to induce many positive effects to protect the body and cells from damage, but improper administration can culminate in hazardous effects, including the stimulation of tumor growth. The studies conducted until now have shown great potential for the clinical use of melatonin to target certain diseases; however, more research must be completed to establish the precise role of melatonin in the body and its range of effects on physiology and behavior.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sánchez-Hidalgo M1, Guerrero JM, Villegas I, Packham G, de la Lastra CA. (2012). Melatonin, a natural programmed cell death inducer in cancer. Curr Med Chem. 2012;19:3805-21
2. Wang J1, Xiao X, Zhang Y, Shi D, Chen W, Fu L, Liu L, Xie F, Kang T, Huang W, Deng W. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53:77-90
3. Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179-88
4. Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Medicine Reviews. 2009;13:257-64
5. Agrawal A, Darbari S, Rai TP, Kulkarni GT. Role of Melatonin in the Pathophysiology of Cancer. J Chron DD. 2016;7:1-6
6. Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J. Pineal Res. 1993;14:151-68
7. Cutando A1, López-Valverde A, Arias-Santiago S, DE Vicente J, DE Diego RG. Role of Melatonin in Cancer Treatment. Anticancer Res. 2012;32:2747-53
8. Regestein QR, Pavlova M. Treatment of delayed sleep phase syndrome. Gen Hosp Psychiatry. 1995;17:335-45
9. Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11-24
10. Schomerus C, Korf H-W. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N.Y. Acad. Sci. 2005;1057:372-83
11. Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system". Ann. N.Y. Acad. Sci. 2001;939:200-15
12. Reiter RJ1, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129-46
13. Carrillo-Vico A, Lardone P. J, Álvarez-Sánchez, N, Rodríguez-Rodríguez A, & Guerrero JM. Melatonin: Buffering the Immune System. International Journal of Molecular Sciences. 2013;14:8638-83
14. Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10:467-76
15. Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Melatonin administration attenuates depressed immune functions trauma-hemorrhage. J. Surg. Res. 1996;63:256-62
16. Morrey KM, McLachlan JA, Serkin CD, Bakouche O. Activation of human monocytes by the pineal hormone melatonin. J. Immunol. 1994;153:2671-80
17. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25-39
18. Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals - a unifying hypothesis. J. Endocrinol. 2003;179:1-13
19. Rondanelli M, Faliva M. A, Perna, S, & Antoniello, N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: review and remarks. Aging Clinical and Experimental Research. 2013;25:499-510
20. Wang YM, Jin BZ, Ai F. et al. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69:1213-20
21. García-Santos G, Antolín I, Herrera F, Martín V, Rodriguez-Blanco J, del Pilar Carrera M, Rodriguez C. Melatonin induces apoptosis in human neuroblastoma cancer cells. J Pineal Res. 2006;41:130-5
22. Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97:1084-7
23. Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. J Natl Cancer Inst. 2004;96:475-82
24. Verkasalo PK, Lillberg K, Stevens RG. et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595-600
25. Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66:5521-5
26. McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15:241-9
27. Perreau-Lenz S1, Pévet P, Buijs RM, Kalsbeek A. The biological clock: the bodyguard of temporal homeostasis. Chronobiol Int. 2004;21:1-25
28. Do MTH, Yau K-W. Intrinsically Photosensitive Retinal Ganglion Cells. Physiological Reviews. 2010;90:1547-81
29. Paul KN, Saafir TB, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Reviews in Endocrine & Metabolic Disorders. 2009;10:271-8
30. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001;535(Pt 1):261-7
31. Malhotra S, Sawhney G, Pandhi P. The therapeutic potential of melatonin: A review of the science. Medscape General Medicine. 2004;6:46
32. Bauer BA. Melatonin side effects: What are the risks?. https://forum.psychlinks.ca/showthread.php?32015-Melatonin-side-effects-What-are-the-risks
33. Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177-95
34. Wiechmann AF1, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137-60
35. Tosini G1, Fukuhara C. Photic and circadian regulation of retinal melatonin in mammals. J Neuroendocrinol. 2003;15:364-9
36. Petrie K, Dawson AG, Thompson L, Brook R. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol Psychiatry. 1993;33:526-30
37. Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001;86:4727-30
38. Blask D, Dauchy R, Dauchy E, Mao L, Hill S, Greene M, Belancio V, Sauer L, Davidson L. Light Exposure at Night Disrupts Host/Cancer Circadian Regulatory Dynamics: Impact on the Warburg Effect, Lipid Signaling and Tumor Growth Prevention. PLoS ONE. August 6. 2014 https://doi.org/10.1371/journal.pone.0102776
39. Boeve BF1, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281-4
40. Pavel S, Goldstein R, Petruscu M. Vasotocin, melatonin and narcolepsy: possible involvement of the pineal gland in its patho-physiological mechanism. Peptides. 1980;1:281-4
41. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol (Meta-analysis). 2011;53:783-92
42. Bartsch C, Bartsch H, Jain AK, Laumas KR, Wetterberg L. Urinary melatonin levels in breast cancer patients. J Neural Transm. 1981;52:281-94
43. Keifer T, Ram PT, Yuan L, Hill SM. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res. Treat. 2002;71:37-45
44. Anisimov VN, Alimova IN, Baturin DA. et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer. 2003;103:300-5
45. Kaczor T. An overview of melatonin and breast cancer. Nat Med Journal. 2010;2:2
46. Bartsch C, Bartsch H, Fluchter St H, Lippert TH. Depleted pineal melatonin production in primary breast and prostate cancer is connected with circadian disturbances: possible role of melatonin for synchronization of circadian rhythmicity. In: (ed.) Touitou Y, Arendt J, Pevet P. Melatonin and the Pineal Gland - From Basic Science to Clinical Application. New York: Elsevier. 1993:311-316
47. Khoory R, Stemme D. Plasma melatonin levels in patients suffering from colorectal carcinoma. J Pineal Res. 1988;5:251-8
48. Kvetnaia TV, Kvetnoy IM, Bartsch H. et al. Melatonin in cancer with extra-reproductive location. Bartsch C, Bartsch H, Blask DE, et al, eds. The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy Berlin: Springer 2001: 177-196
49. Viviani S, Bidoli P, Spinazze S. et al. Normalization of the light/dark rhythm of melatonin after prolonged subcutaneous administration of interleukin-2 in advanced small cell lung cancer patients. J Pineal Res. 1992;12:114-7
50. Dogliotti L, Berruti A, Buniva T. et al. Melatonin and human cancer. J Steroid Biochem Mol Biol. 1990;37:983-7
51. Luboshitzky R, Shen-Orr Z, Nave R, Lavi S, Lavie P. Melatonin administration alters semen quality in healthy men. J Androl. 2002;23:572-8
52. Gwayi N, Bernard RT. The effects of melatonin on sperm motility in vitro in Wistar rats. Andrologia. 2002;34:391-6
53. Puig-Domingo M, Webb SM, Serrano J, Peinado MA, Corcoy R, Ruscalleda J, Reiter RJ, de Leiva A. Brief report: melatonin-related hypogonadotropic hypogonadism. N Engl J Med. 1992;327:1356-9
54. Van Geijlswijk IM, Mol RH, Egberts TCG, Smits MG. Evaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology. 2011;216:111-20
55. Bubenik GA, Blask DE, Brown GM, Maestroni GJ, Pang SF, Reiter RJ, Viswanathan M, Zisapel N. Prospects of the clinical utilization of melatonin. Biol Signals Recept. 1998;7:195-219
56. Silman RE. Melatonin: a contraceptive for the nineties. Eur J Obstet Gynecol Reprod Biol. 1993;49:3-9
57. Wiechmann AF, O'Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 1992;33:1894-902
58. Penn State Hershey. Possible Interactions with: Melatonin. http://pennstatehershey.adam.com/content.aspx?productId=107&pid=33&gid=000970
59. University of Maryland Medical Center. Possible Interactions with: Melatonin. https://www.umm.edu/health/medical/altmed/supplement-interaction/possible-interactions-with-melatonin
60. Konturek SJ, Konturek PC, Brzozowski T. 2006. Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J Physiol Pharmacol. 2006;57(Suppl 5):51-66
61. Kruse J. REALITY #4: WHY EYE AND BRAIN DISEASES ARE EXPLODING. https://www.jackkruse.com/reality-4-eye-brain-diseases-exploding/
62. Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, Dauchy EM, Frasch T, Duplesis T. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235-45
63. Buscemi N, Vandermeer B, Pandya R. et al. Melatonin for Treatment of Sleep Disorders. AHRQ Evidence Report Summaries. 2004: (MD) Agency for Healthcare Research and Quality (US). 1998 -2005. 108
64. Khoury J, Doghramji K. Primary Sleep Disorders. Psychiatr Clin North Am. 2015;38(4):683-704
65. Thorpy M. Classification of Sleep Disorders. Neurotherapeutics. 2012;9:687-701
Author contact
![]() Corresponding author: Yi Lu, Ph.D., Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, 19 South Manassas Street, Memphis, TN 38163 (USA). Tel.: (901) 448-5436; Fax.: (901) 448-5496; E-mail: yluedu
Corresponding author: Yi Lu, Ph.D., Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Cancer Research Building, 19 South Manassas Street, Memphis, TN 38163 (USA). Tel.: (901) 448-5436; Fax.: (901) 448-5496; E-mail: yluedu